Sinopharm Vaccine European Approval - The Czech Republic Becomes The Second Eu Country To Ask For China S Sinopharm Vaccine
BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or. Hungary signs deal for Chinese Sinopharms COVID-19 vaccine first in EU.
Hungary Becomes First In Eu To Authorize Sinopharm Covid Vaccine Bloomberg
The World Health Organisation approved Chinas Sinopharm vaccine for emergency use in May.
Sinopharm vaccine european approval. The Sinopharm vaccine is approved by the following eight countries. As for the Sinopharm and Sinovac vaccines they are both recognised by eight European countries. Cyprus on Thursday added Chinese Covid vaccine Sinopharm to the list of approved vaccines accepted for entry into the country.
A number of African Latin American and Asian countries have chosen to approve and use the Vero Cell vaccine by the Beijing-based pharmaceutical company Sinovac and. EMA is in charge of the. The UAEs business and expat community sighed with relief as it was.
On 7 May 2021 the World Health Organization approved the vaccine for use in COVAX. It will be the first vaccine to carry vaccine vial monitors - small stickers that will change. Xinhua The first Chinese COVID-19 vaccine plant in Europe the.
A medical worker poses with a vial of the Sinopharms COVID-19 vaccine. Photo taken on June 1 2021 shows a vial of the Sinopharm vaccine in Beijing capital of China. Serbia which already has a factory to produce Russias Sputnik V vaccine is set to become the first European country to make the Sinopharm vaccine too.
Although the Sinopharm vaccine has not been approved by the European Medicines Agency Serbia bought four million doses and received another 200000 as a donation. Sinopharm got the WHOs approval on 7 May 2021 for emergency use giving the green light for this vaccine to be rolled out globally. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the.
Hungary in particular bypassed the European Commissions bulk purchasing mechanisms to secure its own supplies of Sputnik V and Sinopharm. As a large share of the European Union and Schengen Area countries have opened their borders for travellers who can prove that they have been fully vaccinated against. You have to carry documentation of a negative test anyway if you are not vaccinated with an EMA approved vaccine but you will still have to quarantine for 10 days.
A further 42 countries including Hungary Venezuela and Sri Lanka have approved the vaccine. The decision comes into force on. WHO recommends the vaccine for.
Why WHO approval of Sinopharm vaccine will boost European coffers from Middle East travel. Vaccine which has been widely used across the region is expected to be. EMA approval does not.
COVID-19 vaccines authorised for use in the EU following evaluation by EMA with links to detailed information on each authorised vaccine. What you need to know. The Sinopharm COVID-19 vaccine.
However the European Medicines Agency EMA has not yet reviewed. Decisions about which COVID-19 vaccines are included for example in the EU Digital COVID Certificate are taken by the EU Member States. The Sinopharm vaccine is approved by the following eight.

Serbia To Start Producing Chinese Sinopharm Vaccine Euractiv Com
The Geopolitics Of Covid Vaccines In Europe S Eastern Neighbourhood European Council On Foreign Relations

Who Approves Chinese Covid Vaccine For Emergency Use Worldwide Voice Of America English

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021

Travellers Vaccinated With Russian Chinese Indian Vaccines May Be Unable To Enter Majority Of Eu Countries Schengenvisainfo Com
Hungary Reaches Deal To Buy China S Sinopharm Vaccine Pm Aide Says Reuters

Hungary Rolls Out China S Sinopharm Jab Amid Lagging Trust
Eu S Covid Travel Pass Will Let Countries Choose Russia China Vaccines Politico
China Helps Serbia Surge Ahead In European Vaccine Race

European Commission Buys Additional Moderna S Covid 19 Vaccine Doses
Cansino Granted Gmp Certificate From Hungary After Sinopharm Increasing Eu Confidence In Recombinant Covid 19 Vaccine Global Times

The Czech Republic Becomes The Second Eu Country To Ask For China S Sinopharm Vaccine

Eu Travel Covishield Sinopharm Sinovac Vaccines Are Most Widely Accepted By Eu Countries After Those Authorised By Ema Schengenvisainfo Com

Eu Approves Second Coronavirus Vaccine

Russia S Sputnik Vaccine Gets Its First Approval In The Eu Uae
Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
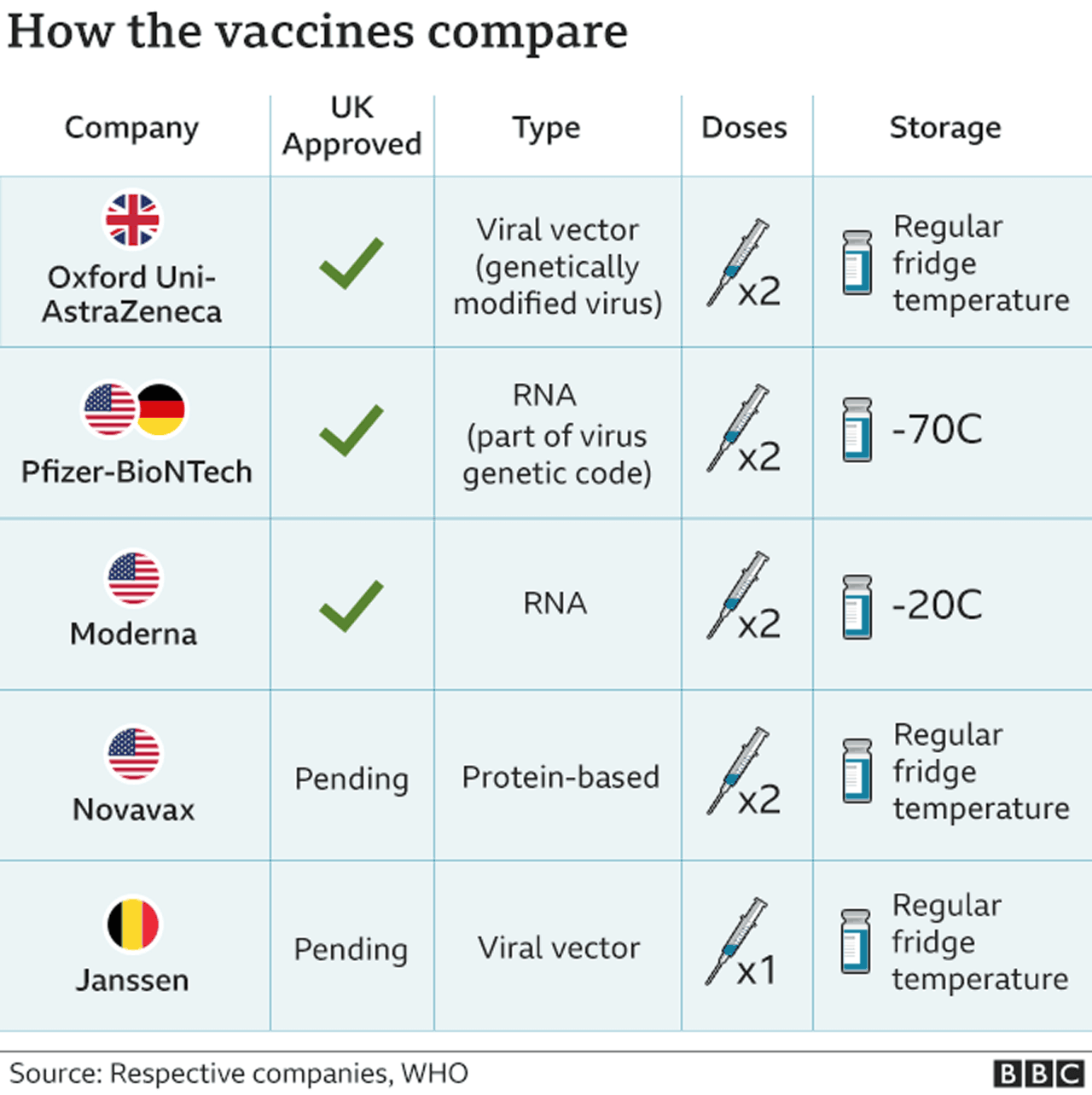
Eu Unveils Plans For Overseas Tourists To Return Bbc News

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
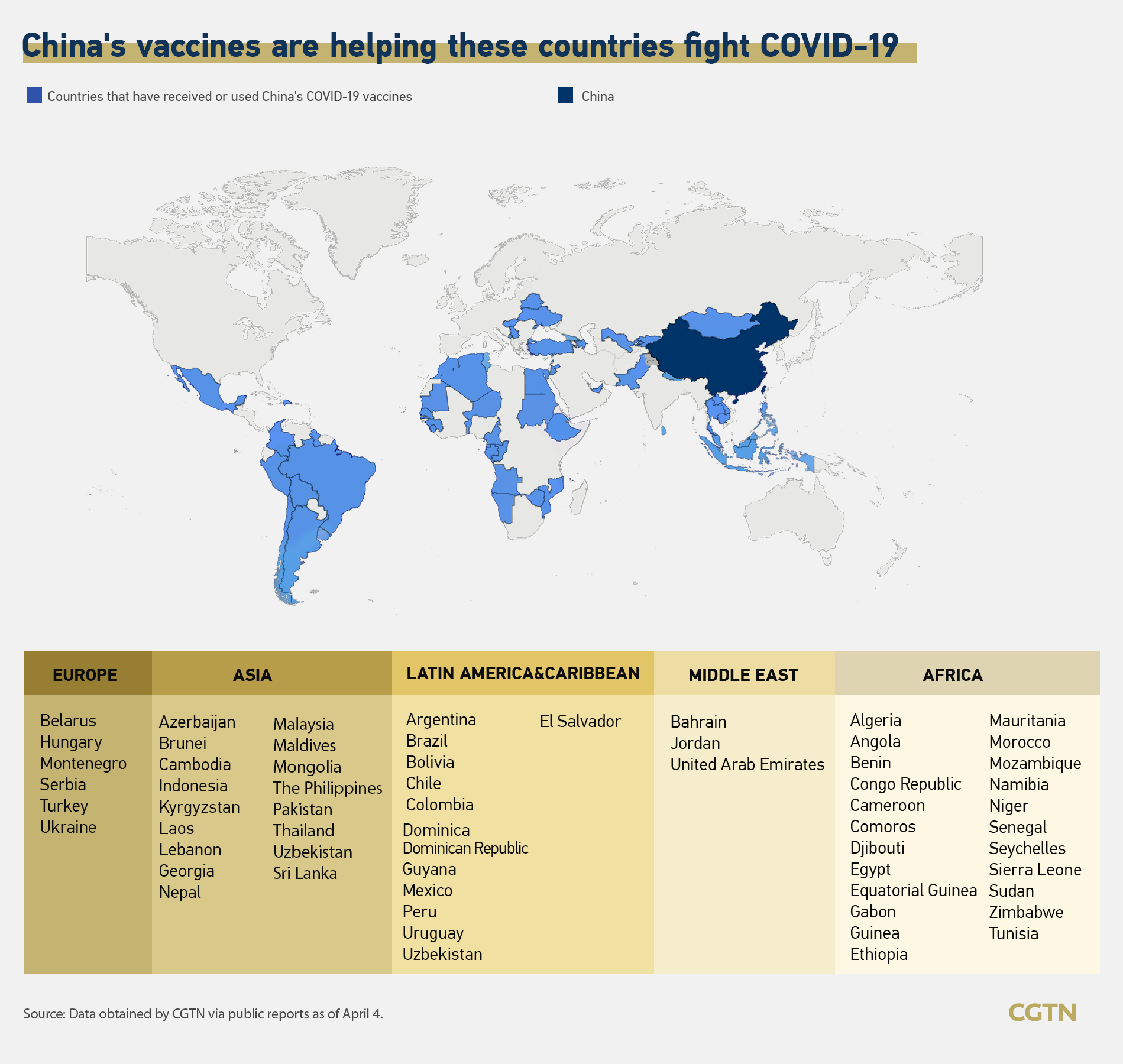
Covid 19 China Vaccines Aid More Nations With 1st Eu Gmp Certificate Cgtn